As a typical multifunctional material, diamond possesses numerous advantages such as high hardness, high thermal conductivity, high stability, corrosion resistance, and excellent biocompatibility. Pure diamond is non-conductive, while boron-doped diamond films exhibit properties of semiconductors or even low-temperature superconductors depending on the boron doping concentration. Boron-doped diamond films also have significant advantages in the field of electrochemistry, including a wide potential window, low background current, and high electrochemical stability, and are widely recognized as excellent electrochemical electrode materials.
The introduction of impurities into diamond enables the acquisition of additional properties while retaining its inherent excellent characteristics. For instance, pure diamond is non-conductive and classified as an insulator; however, the incorporation of trace impurity elements improves its electrical conductivity, endowing it with the potential to serve as a semiconductor material. Boron, with an atomic radius smaller than that of carbon, can easily enter the diamond lattice and replace some carbon atoms. Boron-doped diamond shows substantial enhancements in conductivity, oxidation resistance, corrosion resistance, and heat resistance.
In the undoped state, carbon atoms on the surface of the diamond crystal have one extra valence electron, which may form bonds with electron-deficient external substances, thereby reducing the oxidation resistance of diamond. When boron atoms are doped, boron-carbon covalent bonds are formed, which enhance the chemical inertness of diamond, leading to better oxidation resistance and corrosion resistance. For example, the oxidation resistance temperature of boron-doped diamond is approximately 200–250 °C higher than that of undoped diamond.
Compared with pure diamond, boron-doped diamond exhibits significant differences in electrical and electrochemical properties. Pure diamond is an insulator with a resistivity of up to 10¹⁶ Ω·cm. Boron can greatly improve the electrical conductivity of diamond because each boron atom can only provide 3 electrons to form covalent bonds with adjacent carbon atoms; the unpaired electron from one carbon atom thus forms a hole, resulting in a p-type semiconductor structure. Boron atoms form impurity energy levels in diamond, and the distance between these impurity levels and the valence band is much smaller than the band gap of diamond. Consequently, holes in the impurity energy levels can be easily excited by ionization to enter the valence band, thereby making diamond conductive [13-14]. By adjusting the boron doping concentration, diamond can exhibit properties of semiconductors, conductors, or even superconductors at low temperatures.
Diamond, with its wide band gap and high thermal conductivity, is known as the "ultimate semiconductor material." As mentioned earlier, diamond needs to be doped with impurity elements to achieve semiconductor properties. A high boron doping concentration can cause significant lattice distortion in diamond grains; annealing at 1000 °C can restore the lattice integrity of nanodiamond, reduce internal stress caused by doping, and improve the electrical properties of the film. All the aforementioned research works have successfully obtained boron-doped diamond with resistivity consistent with the characteristics of p-type semiconductors.
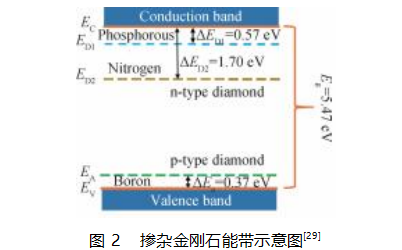
N-type diamond semiconductors are mainly nitrogen-doped. The impurity energy level of n-type donors is deep, reaching 1.7 eV. However, the solid solubility of n-type acceptor impurities in diamond is low; nitrogen has a deep energy level in the diamond lattice, and nitrogen-containing diamond has high resistivity, which cannot meet the requirements of an n-type semiconductor material. Existing studies have shown that co-doping methods such as boron-phosphorus, boron-sulfur, and boron-nitrogen are promising for obtaining n-type diamond semiconductors with excellent properties.
The boron-doped single-crystal diamond produced by CSMH can achieve doping from low concentration to high concentration. It has realized a uniform and controllable concentration and a customizable boron doping process.CSMH uses the MPCVD method to prepare large-sized and high-quality diamonds,and currently has mature products such as diamond heat sinks, diamond wafers, diamond windows,diamond hetero junction integrated composite substrates,etc
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message