A material’s ability to move heat is measured in thermal conductivity. Thermal conductivity is measured in Watts per meter-Kelvin, W/mK. A material with a conductivity value of 1 W/mK will transfer heat at a rate of 1 watt for every degree Kelvin (or Celsius) of temperature difference across a thickness of 1 meter.
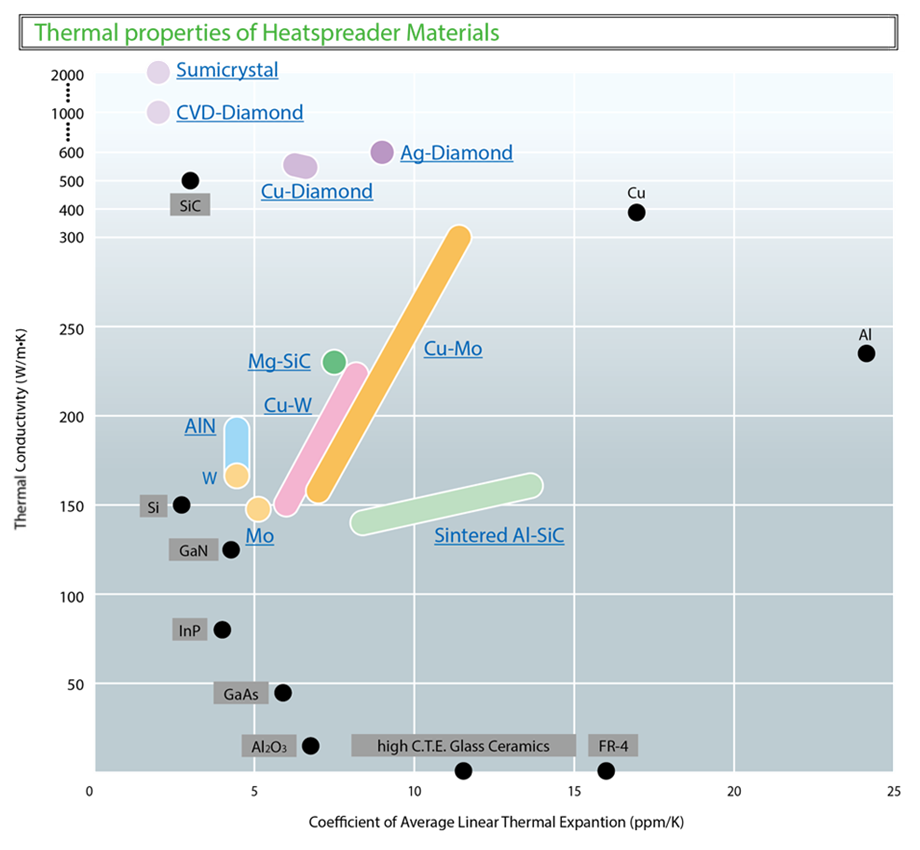
Copper is commonly used to carry away heat from electronic components. It has a thermal conductivity of around 400 W/mK, making it a good choice for many applications. Copper, however, is heavy and can easily tarnish and corrode. It’s not a good material to use for thermal management in harsh environments, or in aerospace where every gram counts. Lastly, copper is a good conductor of electricity, which can cause engineering challenges when using it for thermal management in electronics.
Silicon carbide (SiC) resists many strong acids, bases, oxidants, and chlorinated organics and is used for thermal management in harsh environments. It also does not easily conduct electricity. With a thermal conductivity of 120 W/mK, however, SiC can’t meet copper’s conductivity.
Diamond is a crystalline arrangement of common carbon. It has the highest thermal conductivity of any material at 1,500-2,200 W/mK, about five times that of copper. How does diamond conduct heat so well? The key is in its structure.
Diamond has a cubic crystal structure. Each carbon atom is covalently bonded to four other carbon atoms to form a tetrahedron (pyramid shape). There are no free electrons in this structure, so diamond does not conduct electricity. Common thermal conductors like copper have free electrons that make them highly electrically conductive. They can make use of peripheral electrons for heat transfer. Because diamond isn’t electrically conductive, heat is transferred only through atomic vibrations. The rigid continuous crystal structure of diamond enables these vibrations to travel very quickly through a piece of diamond. And that translates into fast heat conduction.
Purity is key to diamond’s heat conductivity. Impurities in diamonds can slow down or disrupt the spread of lattice vibrations, making it less efficient at conducting heat. (Some impurities can even change the key properties of diamond. For example, natural blue diamond contains boron that makes it a semiconductor.)
Diamond’s crystal structure also make it extremely resistant to acids, bases, oxidants, and other chemicals. It’s the hardest naturally occurring material known. (Harder specialized man-made materials exist, but they don’t share diamond’s other unique properties.)
Diamond is a good material for thermal management in high-performance electronics. Diamond heat spreaders conduct heat much better than copper or aluminum, don’t conduct electricity, are lightweight, resistant to corrosion, and are extremely durable.
There are several reasons to use diamond as a heat conductor instead of other materials like copper, aluminum, or SiC:
1.Thermal conductivity: Diamond has a thermal conductivity between 1,500-2,200 W/mK, the highest known. Copper has a thermal conductivity of 400 W/mK, aluminum 220 W/mK.
2.Thermal stability: Diamond doesn’t expand or contract much at different temperatures, meaning it remains very stable over a wide range of temperatures. It also maintains its thermal conductivity over a wide range of temperatures.
3. Corrosion resistance: Diamond is immune to strong acids, bases, and even organic solvents.
4. Electrical conductivity: Diamond does not conduct electricity and is a very good insulator.
5. Durability: Diamond is one of the hardest materials known.
6. Lightweight: Diamond is lightweight when compared to other common heat-conducting materials like copper.
7. Transparency: Diamond can be transparent.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message