Diamond is a structure of carbon. In a diamond crystal, each carbon atom has 4 nearest neighboring carbon atoms and 12 next neighboring carbon atoms. There are 8 atoms in a unit cube. As the radius of boron atoms is smaller than that of carbon atoms, it is easy to enter the diamond crystal lattice. Boron exists in diamond crystals in three possible forms: one is that boron atoms take the place of carbon atoms; the second is that boron atoms are located in between carbon atoms; and the third is that boron atoms also fill the defects appearing in the growth process of diamond crystals, especially surface defects, and they all have an effect on the performance of diamond crystals.
Boron in diamond crystals can be realized through the addition of boron or borides. The boron content in boron-containing diamond crystals is generally low, but the effect on improving and enhancing the properties of diamond crystals is significant. The results of the study show that the influence of the element boron on diamond is mainly in the following aspects:
Conductivity
The crystal structure of diamond is typical of an atomic lattice. Typical atomic lattices have no free electrons present and are therefore non-conductive. Ordinary diamond is an insulator not only because it has four valence electrons, but also a large forbidden band width of about 5.5 electron volts.
It has been proven that trace amounts of chemical impurities can control the conductivity of semiconductors. For example, if boron is added to silicon in the ratio of 1 boron atom to 105 silicon atoms, the conductivity is increased by a factor of 103 at room temperature because boron, a typical trivalent impurity, can obtain electrons from within the valence band, leaving holes behind.
If a boron atom is introduced into a diamond crystal, the energy band state will change. Because the boron periphery only 3 electrons, in the composition of the valence of health is always missing 1 electron, the formation of a negative electric center, which also produces a hole; due to the introduction of boron atoms, so that the number of holes greatly increased, so its electrical conductivity is also greatly enhanced.
Semiconductor properties
For pure diamond, because it has no free movement of the internal electron and has a wide forbidden band of 5.5ev, so the resistivity is very high, can be used as a good electrical insulator; however, when diamond doped into the Ⅲ or Ⅴ elements, diamond can be transformed into a semiconductor or even a conductor from the insulator. With three valence electrons of boron atoms into the diamond lattice will replace the carbon atom in the form of substitution to become the main center, the lattice produces hole carriers, diamond becomes a hole semiconductor, this doping is called P-type doping. With the increase of boron content, the conductivity of diamond increases. It has great prospects for development in the application of high temperature and high power electronic devices.
Oxidation Resistance
The oxidation resistance of boron containing diamonds is better than that of ordinary diamonds and increases with the increase of boron content in them. The main structure of boron-containing diamond is similar to that of ordinary diamond, except that some positions of carbon atoms are replaced by boron atoms, which gives it a different atomic structure. The 3 valence electrons of boron combine with the dangling bonds of carbon atoms to form covalent bonds, making the structure of boron containing diamond without dangling bonds, i.e., the excess valence electrons are combined to form covalent bonds combining boron and carbon atoms and have a stable state, so boron containing diamond has oxidation resistance and heat resistance, and experiments have shown that the surface onset of oxidation temperature of boron containing diamond is higher than that of ordinary diamond by 150℃~250℃.
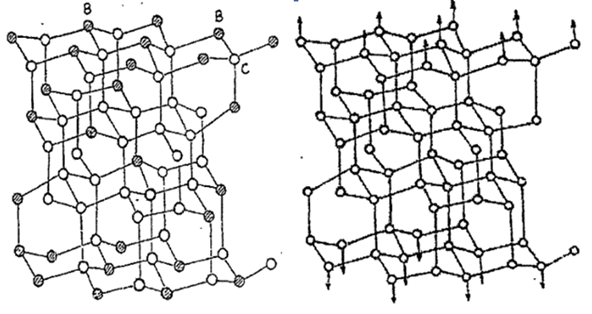
Compressive strength
Artificial diamonds, due to certain reasons during the growth process, result in the existence of bubbles, dislocations, impurity inclusions and other defects, which will result in their mechanical properties being much lower than the theoretical values. Due to the small radius of boron atoms, the chemical activity increases rapidly at high temperatures (1273K). Therefore, it is easier for boron atoms to fill in the defects of diamond during the graphite-to-diamond conversion process; making the structure of diamond denser, which leads to an increase in diamond strength.
In addition to this there is an effect on the following property: chemical inertness. This is expressed here in the size of the affinity of ordinary diamonds and boron-containing diamonds for iron and its alloys, which is generally referred to as adhesion. Boron-containing diamonds are less likely than ordinary diamonds to produce serious adhesion when processing iron and its alloys, resulting in a significant improvement in the quality of the workpiece; color. Diamond is colorless, blue or black due to different concentrations of boron content. Now widely used in semiconductor materials and abrasive materials; impact toughness. Black boron-containing diamond tools have good impact toughness, made of tools in the load intermittent cutting silicon aluminum alloy, titanium alloy, glass steel and other materials with a long life; wear resistance, boron diamond has good wear resistance, suitable for grinding hard and tough materials, but also can be used as a wear-resistant coatings, abrasives, drills, cutting tools and so on.
Responding to the market demand, boron doped single crystal diamond is introduced to meet the needs of the majority of customers in the preparation of high temperature, high power semiconductor components and other applications. The boron doped single crystal diamond produced by CSMH can be doped from low to high concentration, low concentration boron doped diamond has a good mobility, which is suitable for use as the main material for semiconductor devices; high concentration boron doped diamond has a low resistivity, which is suitable for use as electrodes for ohmic contacts.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message