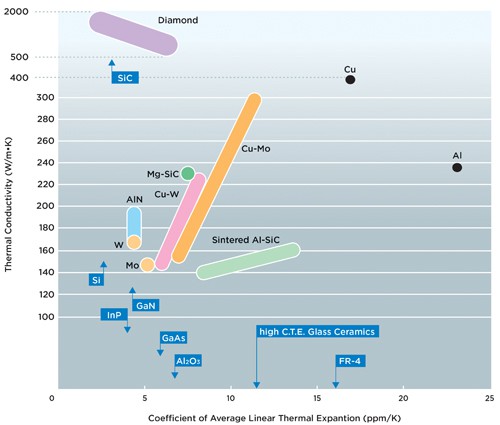
Diamond is one of the best thermal conductors known, in fact diamond is a better thermal conductor than many metals (thermal conductivity (W/m-K): aluminum=237, copper=401, diamond=895). The carbon atoms in diamond are sp3 hybridized and every carbon is bonded to 4 other carbon atoms located at the vertices of a tetrahedron. Hence the bonding in diamond is a uniform, continuous 3-dimensional network of C−C single (sigma) bonds. Graphite on the other hand is formed from sp2 hybridized carbon atoms that form a continuous 2-dimensional sigma and pi bonding network. This 2-dimensional network forms sheets of graphite, but there is little connection between the sheets, in fact, the sheet-sheet separation is a whopping ~3.4 angstroms. This might lead us to suspect that heat conduction in the 2-dimensional sheet of graphite would be superior to diamond, but that heat conduction between graphite sheets would be very low. This is, in fact, an accurate description of thermal conduction in graphite. Thermal conductivity parallel to the graphite sheets=1950, but thermal conduction perpendicular to the sheet=5.7. Therefor, when we consider thermal conduction over all possible directions (anisotropic) diamond would be superior to graphite.
We touch on the mechanisms behind thermal conductivity so we can rationalize the differences between the behaviour of diamond, graphite, and metals:
There are two ways in which heat is transmitted through solids: phonons and electronic conductivity. The latter occurs in electrically conductive solids, where conduction band electrons are free to move throughout the structure, carrying thermal energy along with them. This is a significant component to the thermal conductivity of metals and explains some of the in-plane conductivity of graphite. Electrically conductive solids tend to also be good thermal conductors for this reason, but this mode of conduction is not accessible to diamond.
Phonons are very complicated, but conceptually, phonons are waves of vibrations in the lattice of a solid. (quantized vibrational modes, actually, but not important for this) Vibrational energy in one part of a solid will be transmitted to other parts of the solid as phonons. What limits how quickly heat is transferred by this mechanism is phonon scattering. Phonons can scatter on many different things: crystal defects, impurities, crystal boundaries, even other phonons. This is why crystalline substances (like diamond) tend to have higher thermal conductivity than amorphous ones (like glass or polymers) as the phonons scatter more when they don't have a nice ordered crystal structure to travel through.
The reason why diamond, in particular, is an especially good thermal conductor even compared to other well-ordered crystals boils down to two factors: the mass of the carbon atoms and the strength of the bonds connecting them. I don't want to get too much into the details, but briefly, much as a harmonic oscillator has a higher frequency with a stronger spring (the strength of the C-C bonds) and a lighter mass (the C atoms), diamond can support higher energy phonons. This means that at a given temperature, fewer phonons near the material's limit will be present in diamond than a material with a smaller limit. The details aren't important, but when phonons near the limit interact, they scatter in such a way that the phonons don't travel through the structure. Because diamond's limit is so high, the probability of this type of scattering is lower.
Phonons and electrons travel very well along graphite's graphene sheets, but poorly between them, due to weak inter-layer interactions and the large distance between layers, explaining the anisotropy of its thermal and electronic conductivities.
Metals exhibit both modes of thermal conduction in varying degrees of importance and conduct isotropically since they lack the layered structure of graphite.
Diamond has the highest thermal conductivity of any material, which makes it an excellent choice for thermal management in electronic devices. We provide products and services such as diamond heat sink, wafer-level diamond, GaN on Diamond, Diamond on GaN, etc. Welcome to discuss in detail
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message