Pos:
Home KnowledgeTechnologyCharacterization of Boron-Doped Single-Crystal Diamond in an Oxygen-Enriched EnvironmentAs an extreme functional material, diamond holds great application value in fields such as petroleum exploration, precision instrument processing, medical treatment, and semiconductors. Especially in the semiconductor field, diamond is known as the "ultimate semiconductor material" due to its excellent properties including an ultra-wide band gap (~5.47 eV), high thermal conductivity (120 W/cm·K), and high breakdown electric field (2×10⁷ V/cm).
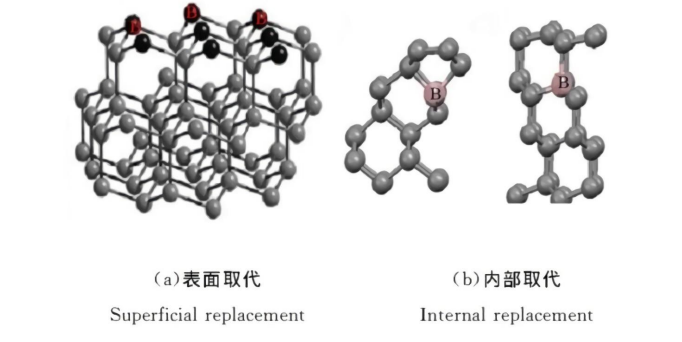
The electrical conductivity of boron-doped diamond can range from highly insulating to nearly metallic. P-type semiconductor devices fabricated using boron-doped diamond or diamond thin films have already been put into practical application. However, due to the lack of n-type semiconductor diamond, it is difficult to fabricate more complex electronic devices using only p-type semiconductor diamond. Studies have shown that introducing oxygen into diamond doping can improve the electrical properties of diamond; n-type conductivity can be achieved by implanting boron and oxygen ions into diamond thin films. Furthermore, in the research on boron-doped single-crystal diamond, it was found that the boron-oxygen composite impurities contained in the crystal surface layer result in n-type conductivity of this layer. These studies indicate that oxygen plays a crucial role in realizing the transition of the electrical properties of boron-doped diamond from p-type to n-type.
Single-crystal diamond was synthesized in the FeNi-C system using amorphous boron as the boron source. The results show that with the increase in boron doping concentration, the crystal morphology transforms from hexa-octahedron to octahedron. When the boron doping concentration is less than 4 wt.%, the growth rate generally shows an increasing trend, but the crystal quality decreases to some extent; when the boron content is 5 wt.% and 6 wt.%, high-quality diamond crystals can no longer be synthesized. Raman spectroscopy results reveal that as the boron doping concentration increases, the peak position (Xc) of the Raman peak shifts toward lower energy, and the full width at half maximum (FWHM) gradually widens. This indicates an increase in internal crystal stress and a decrease in crystallinity. An absorption peak related to the change in the phonon density of states of diamond appears in the Raman spectrum of boron-doped diamond, suggesting that high-concentration boron-containing diamond is synthesized under this doping concentration.
Single-crystal diamond was synthesized in the FeNi-C and FeNi-Ti-C systems with the addition of 0–6 wt.% B₂O₃. The results show that as the B₂O₃ doping concentration increases, the carbon oxides and oxygen in the synthesis chamber lead to an increase in the pressure-temperature (P-T) conditions required for diamond synthesis. Infrared spectroscopy results demonstrate that it is difficult to synthesize boron-containing diamond by adding B₂O₃ to the FeNi-C system, and the addition of B₂O₃ in this system has almost no effect on the nitrogen content inside the diamond. In the FeNi-Ti-C system, the introduction of titanium enables the release of boron and oxygen sources from B₂O₃, allowing boron impurities to be doped into the diamond lattice. X-ray photoelectron spectroscopy (XPS) tests detected boron-oxygen composite impurities on the crystal surface and also identified oxygen-related peaks inside the crystal, indicating that oxygen impurities are doped into the interior of the diamond. Experiments further revealed the mechanism by which titanium reacts with B₂O₃ at high temperatures to generate boron and oxygen impurities. Although the growth of boron-oxygen doped diamond has been initially achieved, the introduction of titanium impurities in the chamber and the fixed ratio of boron to oxygen impurities in B₂O₃ both limit the research on the growth of diamond with adjustable doping ratios of boron and oxygen.
Based on the above research, we further constructed an oxygen-enriched environment for diamond growth in the FeNi-C system by using 3 wt.% Ni₂O₃ as an oxygen-containing additive, and then added different proportions of amorphous boron in this oxygen-enriched environment to regulate the growth of single-crystal diamond. The results show that the synthesized diamonds are all high-quality crystals with complete crystal morphology and minimal surface defects. When the doping concentrations of Ni₂O₃ and boron are 3 wt.% and 5 wt.%, respectively, we successfully synthesized high-quality diamond crystals containing boron-oxygen composite impurities. Electrical tests were conducted on the large-sized (6.5 mm) single-crystal diamond synthesized under this doping concentration using the four-probe method. The results show that the diamond exhibits p-type semiconductor characteristics, with a carrier concentration reaching 2.02×10¹⁸ cm⁻³.
The boron-doped single-crystal diamond produced by CSMH can achieve doping from low concentration to high concentration. It has realized a uniform and controllable concentration and a customizable boron doping process.CSMH uses the MPCVD method to prepare large-sized and high-quality diamonds,and currently has mature products such as diamond heat sinks, diamond wafers, diamond windows,diamond hetero junction integrated composite substrates,etc.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message