Pos:
Home KnowledgeTechnologyStudy on the Oxidation Resistance of Boron-Doped Single-Crystal DiamondIn addition to the properties of conventional single-crystal diamond such as high hardness, high wear resistance, and corrosion resistance, boron-doped single-crystal diamond also has significant advantages in oxidation resistance, chemical inertness, hydrostatic strength, and semiconductor properties. Thus, it is regarded as an ideal material for high-temperature and high-power electronic devices. In recent years, boron-doped single-crystal diamond has been successively fabricated into devices such as photodetectors, field-effect transistors, and electron emission electrodes. Especially the discovery of its superconducting properties has demonstrated the attractive application prospects of boron-doped single-crystal diamond.
Diamond undergoes two high-temperature processes from fabrication to application, and in particular, its operating temperature can reach 1000°C during service. Therefore, the high-temperature oxidation resistance (i.e., high-temperature strength) of diamond is considered one of the most important performance indicators for determining the application fields of diamond.
Researchers at home and abroad have conducted extensive studies on the oxidation process of CVD diamond films. It has been found that the initial oxidation temperature of diamond films is 550°C. Due to the high surface activity and high defect density of the (111) plane, the oxidation reaction preferentially occurs on the (111) plane. The oxidation rate of doped diamond films is one-tenth that of undoped diamond films; the oxidation rate increases with the increase in surface area and decreases with the increase in humidity. Studies suggest that the reduction in oxidation rate is attributed to the barrier effect of the B₂O₃ film formed on the surface of the diamond film during the oxidation process.
The research results on the thermal stability of diamond films induced by vacuum annealing, investigated using high-resolution electron energy loss spectroscopy (HREELS) [13], show that active hydrogen and oxygen atoms possess sufficient energy to strike the hydrogen-terminated surface adsorbates and are simultaneously adsorbed onto the diamond surface to form new chemical bonds.
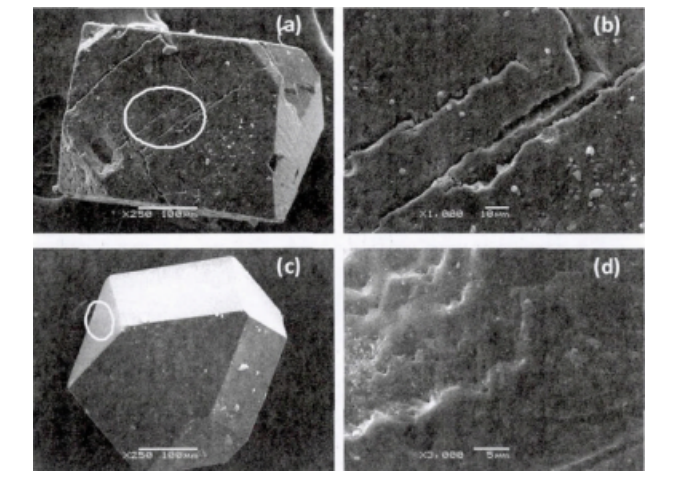
Figure 1 shows the morphologies of conventional single-crystal diamond and boron-doped single-crystal diamond. It can be observed that conventional diamonds are mostly regular hexa-octahedral crystals, presenting a pale yellow color and transparency. In contrast, boron-doped single-crystal diamonds appear blue-black and primarily exhibit an octahedral shape due to the introduction of boron. Studies have confirmed that an increase in boron content leads to a darker color of the diamond crystal, and different crystal faces of the diamond crystal exhibit varying degrees of boron absorption.
For the {111} crystal face, when a boron atom replaces a carbon atom, it forms stable covalent bonds with its three nearest neighboring carbon atoms, as illustrated in Figure 6. The trivalent boron donates all its outer valence electrons to form bonds with carbon atoms, leaving no dangling bonds on the diamond surface. Consequently, no chemical reaction with oxygen occurs. Meanwhile, this substitution also significantly reduces the specific surface energy of the {111} face.
For the {100} crystal face, after a boron atom replaces a surface carbon atom, it bonds with only two of its nearest neighboring carbon atoms. For the trivalent boron atom, this leaves one unpaired electron—i.e., dangling bonds exist on the crystal surface. Therefore, although boron substitution for carbon on the {100} face can also reduce its specific surface energy, the magnitude of this reduction is smaller than that of the {111} face due to the remaining dangling bonds after substitution. In other words, under the same substitution conditions, the {111} face has lower specific surface energy.
Regarding the {100} face, even though boron substitution for carbon leaves dangling bonds on the crystal surface, these dangling bonds, once bonded with oxygen, form B₂O₃ (with a melting point slightly higher than 900 K). This B₂O₃ layer acts as a protective film on the diamond surface, preventing further oxidation. Furthermore, the {100} face does not dominate the surface of the diamond crystal, so its slight oxidation has little impact on the overall thermal stability of the diamond crystal.
Thus, the excellent thermal stability of boron-doped single-crystal diamond should stem from its unique surface crystal structure—specifically, the so-called "boron skin structure" formed when boron atoms replace carbon atoms on the diamond crystal surface.
At different temperatures, both the hydrostatic strength and impact toughness of boron-doped single-crystal diamond are significantly higher than those of conventional single-crystal diamond; moreover, the degree of decrease is small as the temperature rises. This fully indicates that boron-doped single-crystal diamond can maintain high mechanical strength even after being heated.
Differential thermal analysis results show that the initial oxidation temperature of boron-doped single-crystal diamond is approximately 170°C higher than that of conventional single-crystal diamond. Since boron atoms replace some carbon atoms on the surface of the diamond crystal, the oxidation of diamond is effectively prevented or delayed, endowing boron-doped single-crystal diamond with excellent thermal stability.
Experimental results demonstrate that the Fe-Ni-C-B series catalyst prepared by powder metallurgy can synthesize boron-doped single-crystal diamond with outstanding high-temperature oxidation resistance under high-temperature and high-pressure conditions. Furthermore, this preparation method features low cost and good performance, making it suitable for industrial-scale production and application.
The boron-doped single-crystal diamond produced by CSMH can achieve doping from low concentration to high concentration. It has realized a uniform and controllable concentration and a customizable boron doping process.CSMH uses the MPCVD method to prepare large-sized and high-quality diamonds,and currently has mature products such as diamond heat sinks, diamond wafers, diamond windows,diamond hetero junction integrated composite substrates,etc.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message