In brief, heavily boron-doped single crystal SiNMs are first formed on an SOI substrate and then released by selective etching-away of bur- ied oxide. The released SiNMs are transferred to diamond substrate via the stamp-assisted transfer printing method.20 The diamond plate bearing the SiNMs is annealed via RTA to first form a stronger bonding and then to induce boron diffu- sion from Si into diamond. The doping mechanism is described later in the text.
The thermal diffusion doping method has a comparative advantage over ion implantation in that lattice structural damages are not introduced during thermal diffusion. Therefore, the high temperature recrystallization process needed for post ion implantation is no longer necessary and graphitization can be readily avoided. It is also expected that higher crystal quality can be obtained using the thermal dif- fusion method as opposed to the ion implantation method af- ter finishing the doping process. By using the transfer printing method, clean interfaces are ensured and impor- tantly, selective doping (to be seen later), via deterministic transfer printing of SiNMs of different sizes to the selective areas on the diamond surface, is made easy and precise.21 The selective doping enabled by selective transfer printing, while applicable to any size and shape of diamond plates (in contrast to direct wafer bonding), leads to a planar doped structure that can facilitate device implementations. Figs. 1(b) and 1(c) show images of natural diamond before and af- ter SiNM bonding.
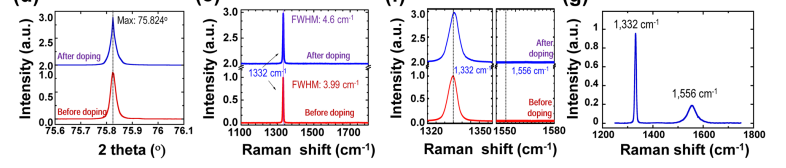
Diamond crystal structures before and after boron diffu- sion doping are first characterized and then compared. Before performing the characterizations, the SiNM is removed using potassium hydroxide (KOH) after completion of the RTA pro- cess. No graphitization removal procedures were applied to the diamond surface. After finishing the Si removal procedure, we are unable to identify any remaining Si or SiC materials in diamond from a Raman spectrum taken in the range of 400– 1200cm-1 (see Fig. S7).43 The X-ray theta-2theta scan results of the boron diffused diamond are shown in Fig. 1(d). The (220) peak of the nSCD, before and after the boron diffu- sion process, appeared at 75.825o in both cases. Of more im- portance, the full width at half maximum (FWHM) of the diamond (220) diffraction peak shows no measurable changes after finishing the diffusion process. The small FWHM value of 0.025o indicates the high single crystallinity of the dia- mond.22,23 The Raman spectra of the nSCD before and after diffusion doping are shown in Fig. 1(e) for comparison. The zoomed-in view of the relevant wave number range of Fig. 1(e) is shown in Fig. 1(f). The sp3 bonding in the sample before and after the thermal diffusion process is clearly indi- cated by the TO phonon peak at 1332cm-1. The FWHM of
the Raman peak became slightly wider and shifted after diffu- sion (from 3.9cm-1 to 4.6cm-1 and 0.4cm-1 of blue-shift). Such a small change could be attributed to the change of the existing crystal imperfection in the nSCD or small stress dur- ing the process. Our nSCD before processing does not have as small FWHM value (2–3cm-1)24–26 in comparison with some of the others that are reported in literature, indicating the exis- tence of some crystal imperfection. However, the FWHM is smaller than 5cm-1 in both cases, further indicating that the sp3 bonding in the diamond remained intact after finishing the boron diffusion process.27,28 It is noted that the FWHM values (13.3cm-1–87.8cm-1)26,29 of Raman spectra in ion implanted (after anneal) SCDs are much larger than 5cm-1.
The X-ray diffraction (XRD) and Raman characteriza- tions indicate that the boron doping method via SiNM bond- ing and thermal diffusion does not induce measurable lattice damage in diamond. Furthermore, as shown in Fig. 1(f) it is noted that the absence of peaks near the wave numbers of 1357cm-1 and 1556cm-1 in the zoomed-in spectra of Fig. 1(e), which are the characteristic indicator of the presence of sp2 bonds, proves that the SiNM doping process does not induce detectable graphitization in the diamond bulk or on its surface. As comparison, Fig. 1(g) shows the Raman spectrum scanned from a reference nSCD sample that has no SiNM bonded but was subject to the identical RTA process. The distinct Raman peak at the wave number of 1556cm—1 in the spectrum clearly indicates the existence of sp2 bonds that are formed on this undoped sample. To further verify the role of SiNM in preventing graphitization on the dia- mond surface, the Raman spectrum taken from the backside of the SiNM bonded diamond (associated with Fig. 1(e) and 1(f)), where no SiNM is bonded, also shows a visible peak at the wave number of 1556cm—1 (see Fig. S3). These results indicate that it is because of using single crystal SiNM as the dopant carrying medium for thermal diffusion that we have successfully avoided graphitization on the diamond surface.
The diffused boron atom concentration in the nSCD is characterized by both secondary ion mass spectroscopy (SIMS) and capacitance-voltage (C—V) measurements. The results are shown in Fig. 2(a) and the fitted curves by the Fick’s law of diffusion are shown in Fig. S4. Fig. 2(a) indi- cates the presence of boron at a concentration of about 1 × 1019 cm—3 at the diamond surface, which is comparable with the level that can be achieved by ion implantation, and gradually decreased to ~2 × 1015 cm—3 at a depth of ~70nm. As a comparison, ~120cm2/v.s of hole mobility and the doping concentration of ~2 × 1018 cm—3 were meas- ured by using Hall measurements (Accent HL5500 Hall sys- tem). Considering that the annealing time is only 40 min, the doping depth achieved is encouraging for device applica- tions. The profile obtained by C—V measurements roughly matches that of SIMS in terms of shape and depth consider- ing the limited accuracy of SIMS. The additional experiment under various diffusion time and temperature conditions is under investigation.
Further characterizations of boron-doped nSCD were performed using Fourier transform infrared spectroscopy (FTIR). Generally, boron inactivation could result from non- substitutional boron sites30 or aggregated substitutional bo- ron sites.31,32 FTIR is an effective method for evaluating the substitutional doping status in a diamond. The FTIR results are shown in Fig. 2(b). Fig. 2(b–i) shows two diamond plates of the same type: one is boron doped (scanning region B) that is realized using the above thermal diffusion method and the other is undoped (scanning region C) as a reference. Figs. 2(b-ii)–(2-iv) show the FTIR mapping results and the scanned spectra. The boron doped diamond shows the char- acteristic absorption peak at 1290cm—1, which clearly indi- cates the electrical activation of boron atoms.33 In contrast, the peak does not appear in the undoped reference diamond. Since substitutional doping, i.e., boron-carbon sp3 bonding formation, is necessary for electrical activation of doped bo- ron atoms, the FTIR and the C—V characterization results prove the substitutional doping of boron atoms in the nSCD. It should be noted that the characteristic absorption peaks associated with boron interstitials and boron interstitial com- plexes in diamond can be observed at 1420, 1530, 1570, and 1910cm—1, but no such peaks appeared in our FTIR spec- tra.34 Moreover, the absence of the three infrared B-B cluster absorption peaks (553, 560, and 570cm—1) indicate that no aggregated substitutional boron sites were formed.35 The broader peaks, which appear from 1900 to 2300cm—1 are the inherent two-phonon lines of diamond associated with C—C bonds. They appear in both the boron doped and undoped di- amond samples.
X-ray photoelectron spectroscopy (XPS) was performed on both doped diamond and undoped diamond (as a refer- ence). The binding energies for Si1s, B1s and C1s peaks have been identified with constant pass energy of 50eV and 100meV energy step as shown in Fig. 2(c). The C–Si peak at ~103 eV indicates that a chemical reaction between Si and carbon atoms has occurred at the Si–diamond interface (Fig. 2(c-i). However, the absence of an Si peak in the Raman spectrum (Fig. S7) in the range of 400–1200cm—1 indicates that the C-Si reaction occurred very shallowly (<10nm) at the diamond surface.43 Boron substitution in diamond yielded a B1s peak at 190.6eV corresponding to B–C (Fig. 2(c-ii)) confirming that boron doping was successful. This is consistent with the SIMS, C—V, and FTIR analyses. Fig. 2(c–iii) shows the XPS spectra for the core C1s peak in the binding energy region around 280–295eV for the undoped and boron doped nSCD samples. De-convoluted C1s peaks using the Gaussian/Lorentzian function in the inset of Fig. 2(c-iii) shows a strong sp3 C–C bonding at 285.4eV and a very small C–O and C = O bonding at 286.4eV and 287.9eV, respectively, indicating that the single crystallinity of nSCD was not degraded by the boron diffusion process.
The XPS results obtained above suggest clues for eluci- dating the boron diffusion doping mechanisms. Especially, the C-Si bonding (Fig. 2(c-i) plays an important role for the observed boron diffusion. First-principles density functional theory (DFT) simulations36,37 were performed to understand the diffusion mechanism. Two mechanisms were proposed to yield enhanced boron diffusion through enhanced vacancies in diamond (detailed calculation can be found in the supple- mentary material). The first mechanism involves injection of excess vacancies into the diamond from the SiNM, which has a much larger intrinsic vacancy concentration than diamond as well as excess vacancies from ion implantation into the SiNM. DFT calculations verify that this vacancy injection is much more energetically favorable than the usual mechanism for vacancy formation in diamond (movement of carbon to the diamond surface). Fig. 2(d) shows the cartoon illustration of the proposed injection and diffusion mechanism. Besides the above vacancy injection into diamond from Si, excess vacancies can also be additionally created by formation of SiC at the SiNM and diamond interface, which stabilized the vacancies by almost exactly the required 0.7 eV needed to explain the enhanced boron diffusion rate into diamond. Both mechanisms could play a role and further research is required to elucidate their contributions. In each case, diffused boron atoms from Si are expected to immediately become substitu- tional atoms in diamond if the diamond does not have pre- existing vacancy related defects (ideal situation). Since no vacancy defects are additionally generated in diamond during the diffusion process, a high temperature anneal that is neces- sary for post-implantation38 then becomes unnecessary in such a doping process.
The boron-doped single-crystal diamond produced by CSMH can achieve doping from low concentration to high concentration. It has realized a uniform and controllable concentration and a customizable boron doping process.CSMH uses the MPCVD method to prepare large-sized and high-quality diamonds,and currently has mature products such as diamond heat sinks, diamond wafers, diamond windows,diamond hetero junction integrated composite substrates,etc.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message