Pos:
Home KnowledgeTechnologyBoron-doped single-crystal diamond is conducive to the improvement of electrical transport propertiesDiamond is an extreme functional material that integrates numerous outstanding properties, including the highest hardness, the highest thermal conductivity, the smallest compressibility, the widest light-transmitting wavelength range, resistance to strong acids and alkalis, and radiation resistance. It has important applications in fields such as quantum information, medical and health care, national defense and military affairs, and industrial technology. Under normal circumstances, both natural diamond and synthetic diamond are insulators. When boron is introduced into the diamond structure, boron acts as a shallow acceptor and can endow diamond with p-type semiconductor properties.
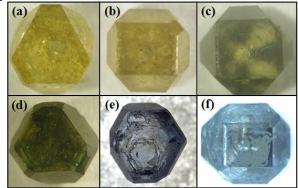
Figs. 1(a) and (b) show the optical photographs of diamonds synthesized without any additives. The two crystals exhibit a typical yellow color, which is caused by nitrogen impurities entering the diamond and occupying carbon atom sites in a single-atom substitutional manner. However, when boron is incorporated into the diamond structure, it causes the diamond to appear blue or even black. Furthermore, boron is more likely to enter the (111) crystal plane than the (100) crystal plane, resulting in a radial distribution of boron in the diamond crystal (as shown in Fig. 1(c)) [11], with the crystal displaying alternating yellow and black colors.If the (111) plane is selected as the crystal growth plane, the (111) crystal plane of the synthesized diamond becomes highly developed while the (100) crystal plane remains very small due to the relatively high synthesis temperature. Under this condition, the distribution of boron in the diamond is relatively uniform, as shown in Fig. 1(d), and the crystal appears dark green. To eliminate the influence of nitrogen impurities on the distribution of boron in the diamond, titanium/copper was used as a nitrogen getter. The purpose of this is to enable the nitrogen impurities inside the synthesis chamber to react with the nitrogen getter to form titanium nitride, which then remains in the molten catalyst. This approach can minimize the incorporation of nitrogen impurities into the diamond.As can be seen from the optical photograph of the crystal in Fig. 1(e), the crystal exhibits a light blue color, and the distribution of boron in this crystal is relatively very uniform. The diamond crystal in Fig. 1(f) has (100) and (111) crystal planes with comparable development degrees; the crystal is generally blue in color, and the distribution of boron in the diamond is extremely uniform.
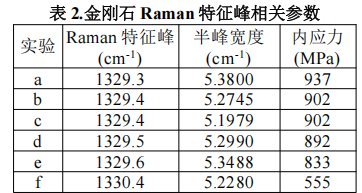
To verify whether the synthesized crystals are of a single sp³-hybridized diamond phase, we conducted Raman spectroscopy tests on the synthesized diamonds, and the test results are presented in Fig. 2. It can be observed that the Raman peaks corresponding to all six diamonds are around 1330 cm⁻¹; the detailed Raman peaks and their corresponding full widths at half maximum (FWHMs) are listed in Table 2. The baseline of the Raman spectra is flat, and there is no graphite peak (1350–1600 cm⁻¹) corresponding to graphite, which indicates that the synthesized crystals are high-quality single-crystal diamonds. Strictly speaking, the FWHM of the diamond Raman peak should be zero. However, due to the presence of impurity defects and internal stress in the diamond structure, the FWHM of the diamond Raman peak is no longer zero.
To characterize the optimized boron distribution in diamond, we conducted Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) tests on the diamond crystal (f), and the test results are shown in Figure 3. In this figure, the orange dots correspond to the concentration of boron (the target element), while the black areas indicate the absence of boron. It can be clearly observed that boron is relatively uniformly distributed on the (100) crystal plane, which will be beneficial for improving the electrical transport properties of diamond.
The boron-doped single-crystal diamond produced by CSMH can achieve doping from low concentration to high concentration. It has realized a uniform and controllable concentration and a customizable boron doping process.CSMH uses the MPCVD method to prepare large-sized and high-quality diamonds,and currently has mature products such as diamond heat sinks, diamond wafers, diamond windows,diamond hetero junction integrated composite substrates,etc.
 闽ICP备2021005558号-1
闽ICP备2021005558号-1Leave A Message